
When fully implemented, the label of most devices will include a unique device identifier (UDI) in human- and machine-readable form, which will ultimately improve patient safety, modernize device postmarket surveillance, and facilitate medical device innovation.
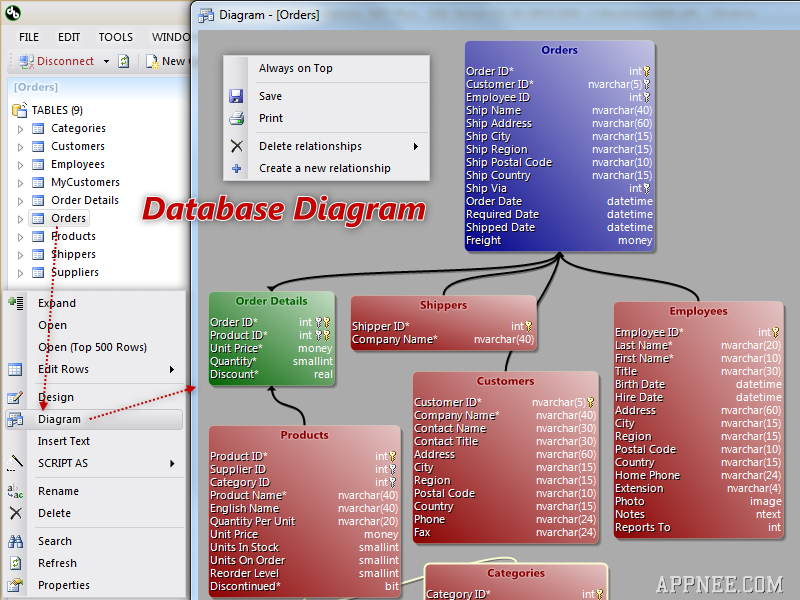
The FDA established the unique device identification system to adequately identify medical devices sold in the United States from manufacturing through distribution to patient use.
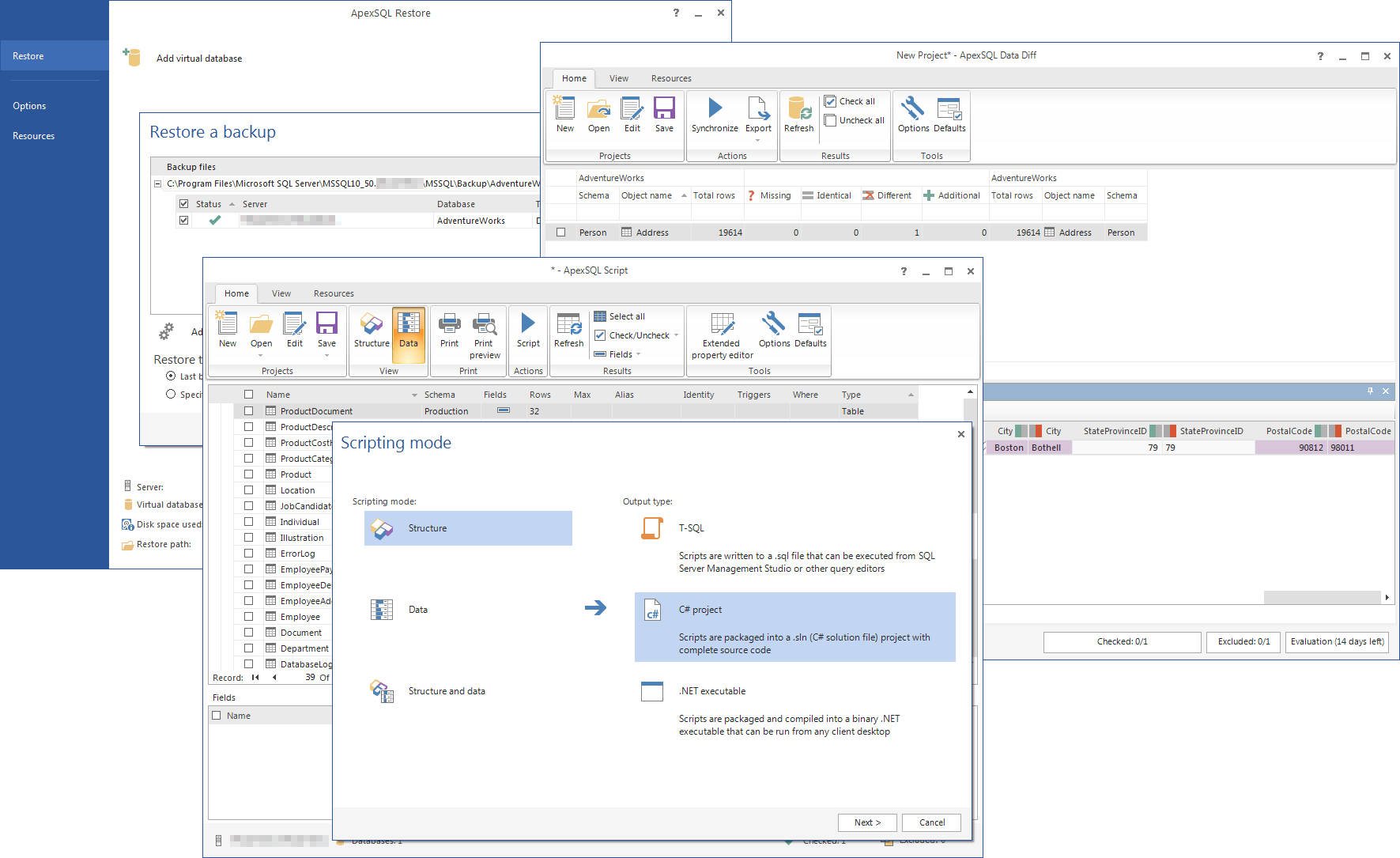
This new date provides a 75-day extension of an existing FDA compliance policy published in the July 2020 version of this guidance. I/LS/LS devices, including Class I I/LS/LS devices, are already expected to comply with GUDID submission requirements. Additionally, the FDA does not intend to enforce the GUDID submission requirements for Class I and unclassified devices, other than implantable, life-supporting or life-sustaining devices, regardless of whether they are consumer health products, before December 8, 2022. CQL programs evolve and migrate data in a mathematically universal way. This final guidance describes the FDA's compliance policy regarding GUDID submission requirements for certain Class I devices considered consumer health products. Open-source CQL and its integrated development environment (IDE) performs. Supports all popular databases: MySQL, PostgreSQL, SQLite, Oracle, DB2, SQL Server, Sybase, MS Access, Teradata, Firebird, Apache Hive, Phoenix, Presto, etc. is a multi-engine database integrated development environment (IDE) designed by.
#UNIVERSAL DATABASE IDE FREE#
Free convenient cross-platform and cross-database Java GUI client.
#UNIVERSAL DATABASE IDE UPDATE#
The update to this guidance reflects the finalization of the draft guidance "Select Updates for Unique Device Identification: Policy Regarding Global Unique Device Identification Database Requirements for Certain Devices", which was issued October 14, 2021, and following the consideration of public comments. Universal Database Tool Free multi-platform database tool for developers, database administrators, analysts and all people who need to work with databases. DBeaver is a free universal database tool and SQL client, built based on. usql is a universal command-line interface for PostgreSQL, MySQL, Oracle Database, SQLite3, Microsoft SQL Server, and many other databases including NoSQL. Universal GUI Tool for Management & Administration, Development for MariaDB and MySQL. FireDAC is a Universal Data Access library for developing applications for multiple devices, connected to.

On July 22, 2022, the FDA posted the final guidance: Unique Device Identification: Policy Regarding Compliance Dates for Class I and Unclassified Devices, Direct Marking, and Global Unique Device Identification Database Requirements for Certain Devices. This final guidance describes the FDA's compliance policy regarding Global Unique Device Identification Database (GUDID) submission requirements for certain Class I devices considered consumer health products. Explore your database direct from the IDE.


 0 kommentar(er)
0 kommentar(er)
